Antibiotic injection recalled after glass fragments found in vials: TFDA
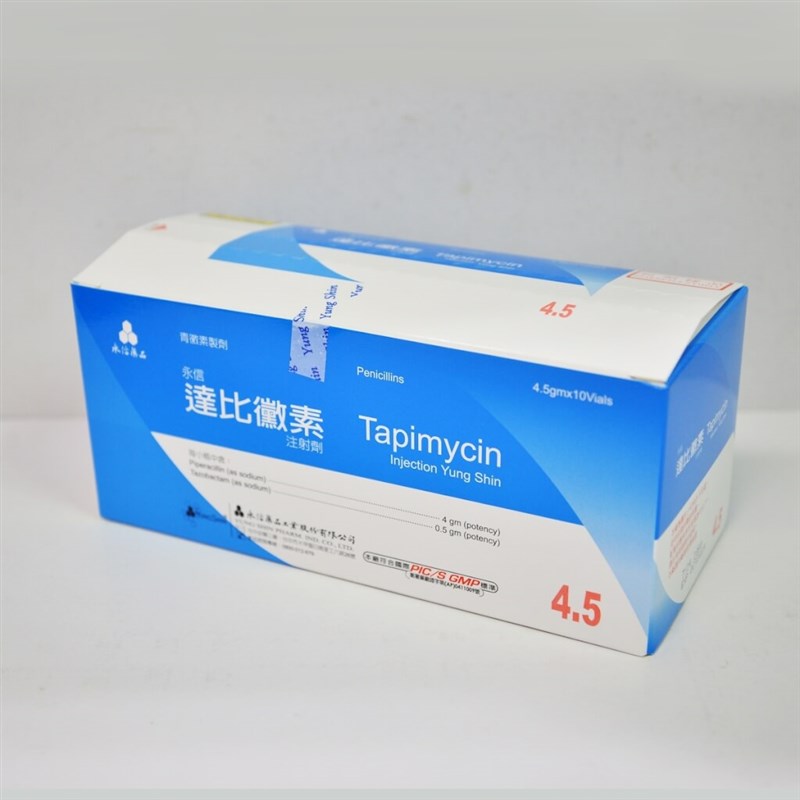
Taipei, Dec. 24 (CNA) Taiwan Food and Drug Administration (TFDA) has ordered the recall of more than 27,000 vials of an antibiotic injection produced by Yung Shin Pharmaceutical Industrial Co. and purchased by medical facilities around Taiwan, after glass fragments were found in one vial, the agency said.
The affected product is Tapimycin Injection Yung Shin, an antibiotic used to treat infections, according to a drug recall notice issued by the TFDA on Monday.
The recall involves a single batch, lot number TYI4 T012, after a hospital reported discovering glass fragments in one vial, prompting the recall, said Huang Mei-chen (黃玫甄), a senior technical specialist with the TFDA.
The batch in question has a shelf life of three years. As of now, a total of 27,680 vials from the batch have already been sold, Huang said. The TFDA has required Yung Shin to complete the recall by Jan. 12, 2026, and to submit a full investigation report along with corrective and preventive action plans.
Huang noted that the same product was recalled in 2021 after glass fragments were found. She said the authorities are still investigating whether the cause of the latest incident is linked to the earlier recall.
Depending on the findings of the investigation, the TFDA may conduct on-site inspections of the manufacturing facility and increase the frequency of regulatory checks, Huang said.
Huang added that the recall is not expected to affect the domestic supply of the medication.
-
Sports
Taiwan men's épée team wins first-ever gold at Asian junior fencing tourney
02/27/2026 09:39 PM -
Politics
Taiwan condemns Beijing over intimidation of interior minister's nephew
02/27/2026 08:23 PM -
Sports
Taiwanese-American Fairchild joins Taiwan at Taipei Dome ahead of WBC
02/27/2026 05:48 PM -
Culture
Young generation carries 228 Incident memories 79 years after massacre
02/27/2026 04:50 PM -
Society
Taiwan records first measles cluster of the year
02/27/2026 04:33 PM