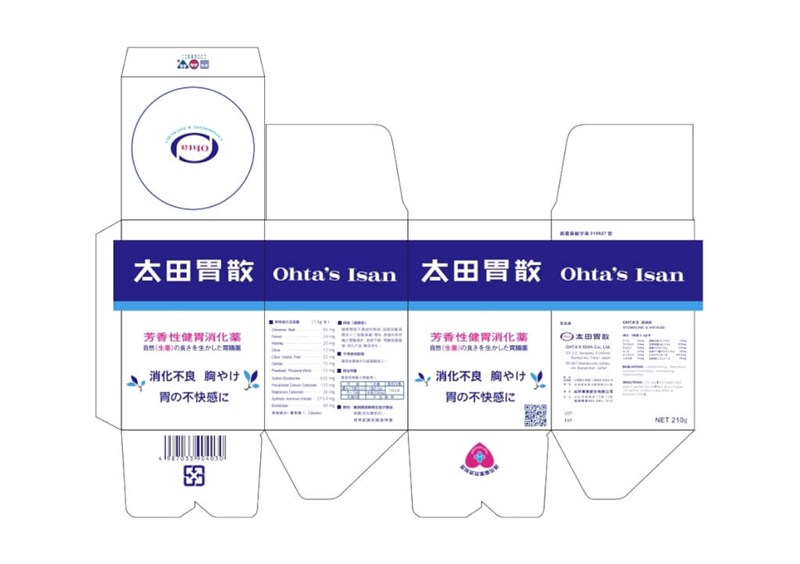
Taipei, Aug. 26 (CNA) Taiwan's Food and Drug Administration (FDA) has ordered a recall of 410,000 cans of the popular Japanese indigestion medicine Ohta's Isan after discrepancies were found between its Chinese and Japanese ingredient labels.
Thirty-seven batches across three product sizes are being recalled by Sept. 18, after a consumer reported that the Japanese labeling of active ingredients on the outer packaging did not match the approved Chinese version, the FDA said Tuesday.
The affected batches include 16 batches of the 75 gram product, 16 batches of the 140 g product, and five batches of the 210 g product, according to the agency.
FDA official Yang Po-wen (楊博文) said the product, imported by Yuo Shiang Industrial Co., is an over-the-counter drug approved with Chinese labeling. Because the Japanese labeling was inconsistent, the FDA ordered a recall.
The distributor must complete the recall and submit a corrective report by Sept. 18, or face fines of NT$200,000 (US$6,550) to NT$5 million under the Pharmaceutical Affairs Act, Yang said.
In addition, the labeling inconsistency may constitute a violation of Article 46 of the act, punishable by fines of NT$30,000 to NT$2 million, he said.
- Business
Corolla Cup shows 'motorsport culture' revving to go: Toyota chairman
09/28/2025 08:37 PM - Society
Families of those killed in Hualien floods to get NT$1 million each: Premier
09/28/2025 06:38 PM - Society
Barrier lake evacuation alerts to be raised to higher emergency level
09/28/2025 05:49 PM - Politics
Ex-submarine program head resigns from National Security Council post
09/28/2025 04:44 PM - Society
Over 24,000 swimmers participate in annual Sun Moon Lake swim fest
09/28/2025 02:44 PM