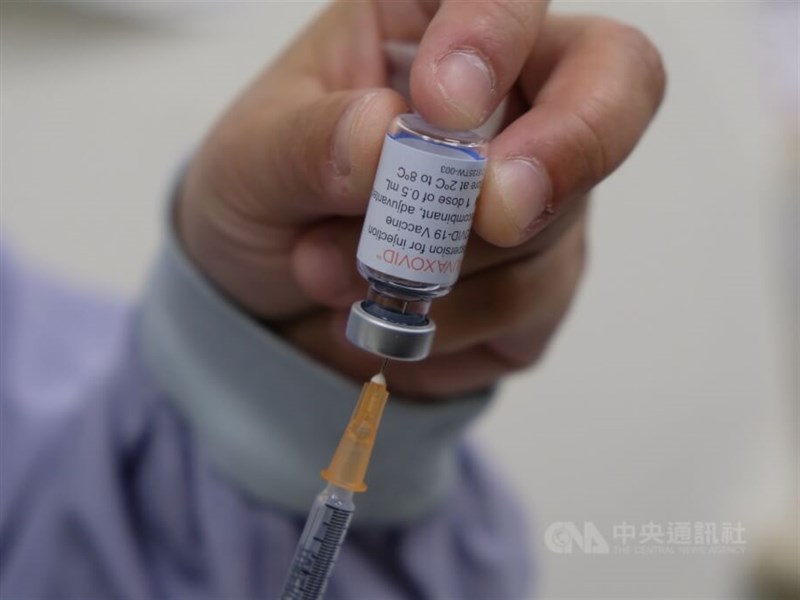
Taiwan's Food and Drug Administration (FDA) has granted emergency use authorization (EUA) for Moderna's Spikevax XBB.1.5 COVID-19 vaccine and is planning to roll out the new jab by the end of this month.
(Full text of the story is now in CNA English news archive. To view the full story, you will need to be a subscribed member of the CNA archive. To subscribe, please read here.)
More in CORONAVIRUS
![Taiwan to shorten COVID-19 vaccine interval to 2 months for high-risk groups]() Taiwan to shorten COVID-19 vaccine interval to 2 months for high-risk groupsThe Centers for Disease Control (CDC) announced Thursday that the required interval between two COVID-19 vaccine doses for high-risk groups will be shortened from six months to two months, effective next Wednesday.06/06/2025 08:17 PM
Taiwan to shorten COVID-19 vaccine interval to 2 months for high-risk groupsThe Centers for Disease Control (CDC) announced Thursday that the required interval between two COVID-19 vaccine doses for high-risk groups will be shortened from six months to two months, effective next Wednesday.06/06/2025 08:17 PM![CDC confirms ample supply of COVID-19 drugs, vaccines]() CDC confirms ample supply of COVID-19 drugs, vaccinesThe Centers for Disease Control (CDC) reiterated Sunday that Taiwan has sufficient antiviral medication for COVID-19 patients and emphasized the importance of vaccination, amid concerns raised by public figures.06/01/2025 06:13 PM
CDC confirms ample supply of COVID-19 drugs, vaccinesThe Centers for Disease Control (CDC) reiterated Sunday that Taiwan has sufficient antiviral medication for COVID-19 patients and emphasized the importance of vaccination, amid concerns raised by public figures.06/01/2025 06:13 PM![CDC issues COVID-19 warning as Dragon Boat Festival long weekend begins]() CDC issues COVID-19 warning as Dragon Boat Festival long weekend beginsMembers of the public should not let their guard down on COVID-19 and seek medical help if they experience symptoms that indicate risk of severe infection, the Centers for Disease Control (CDC) said Friday, the first day of the Dragon Boat Festival long weekend in Taiwan.05/30/2025 05:04 PM
CDC issues COVID-19 warning as Dragon Boat Festival long weekend beginsMembers of the public should not let their guard down on COVID-19 and seek medical help if they experience symptoms that indicate risk of severe infection, the Centers for Disease Control (CDC) said Friday, the first day of the Dragon Boat Festival long weekend in Taiwan.05/30/2025 05:04 PM
Latest
- Society
Taipei knife attack reveals gaps in Metro police deployment
12/26/2025 06:19 PM - Business
U.S. dollar closes lower on Taipei forex market
12/26/2025 04:41 PM - Business
Taiwan shares end higher, led by large cap tech stocks
12/26/2025 03:56 PM - Politics
Legislature passes motion to start impeachment proceedings against Lai
12/26/2025 03:42 PM - Society
Former executive of green energy group indicted on corruption charges
12/26/2025 03:19 PM