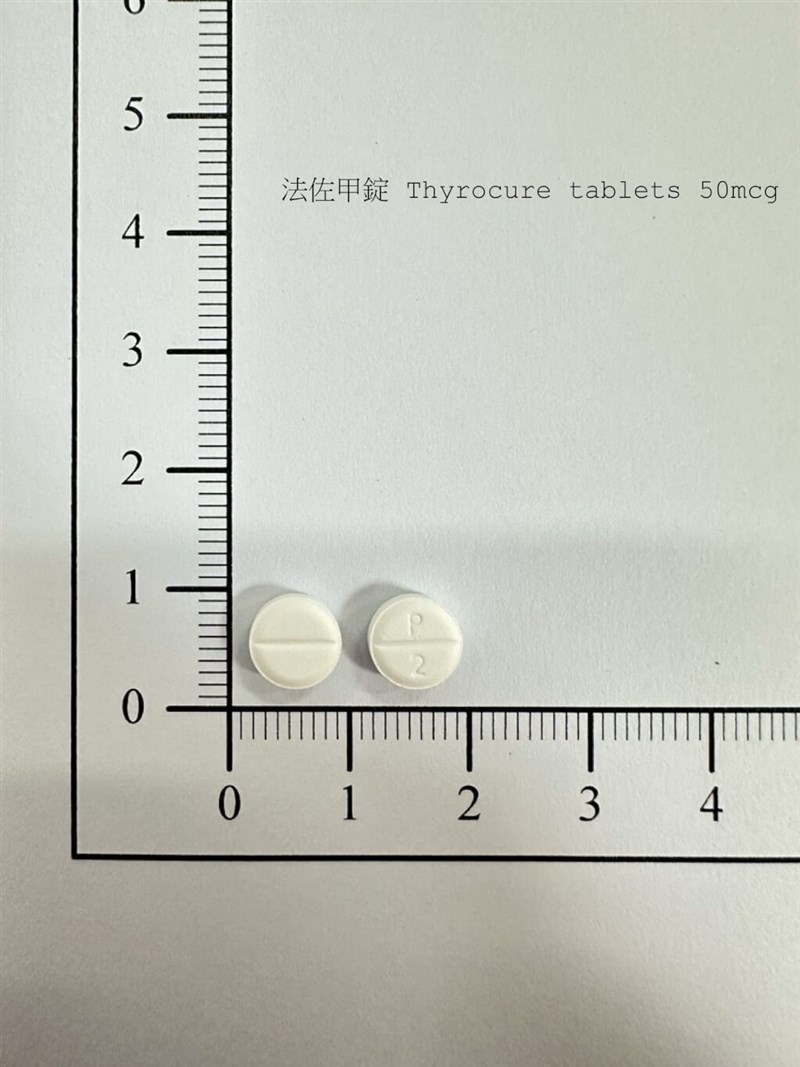
Taipei, Oct. 16 (CNA) Taiwan's Food and Drug Administration (FDA) has ordered the recall of two batches of a medication used to treat hypothyroidism after stability tests found its active ingredient levels had fallen below standard, potentially reducing the drug's effectiveness, an FDA official said Thursday.
The recall covers batches D2402370 and D2402371 of Thyrocure 50 mcg, totaling more than 1.12 million tablets manufactured by Biofrontier Inc., Asia. The medication's main active ingredient, levothyroxine sodium, is used to treat hypothyroidism, or underactive thyroid, according to the FDA.
FDA official Yang Po-wen (楊博文) told CNA that the company voluntarily notified the agency on Sept. 30 after three-month stability tests showed the active ingredient content had fallen below the required specification limit, possibly reducing the drug's effectiveness.
The FDA has instructed the company to complete the recall by Nov. 3, Yang said.
A total of 1,123,000 tablets from the affected batches have been distributed, while 1,175,760 tablets remain in stock -- enough for about 49 months of domestic supply, Yang added. The company also plans to import another 500,000 tablets, or around 21 months of supply, in the first quarter of 2026.
-
Business
Taiwan pineapples set to enter U.S. market under new rules
03/04/2026 08:21 PM -
Society
15-meter whale carcass found in Hualien's Chongde Bay
03/04/2026 07:49 PM -
Society
Taiwan indicts dozens in NT$10.7 billion money laundering case
03/04/2026 07:32 PM -
Society
Taipei Azalea Festival to open Friday with 200,000 blooms
03/04/2026 07:13 PM -
Politics
TPP, KMT to announce joint policy platform on March 13: Huang
03/04/2026 06:57 PM