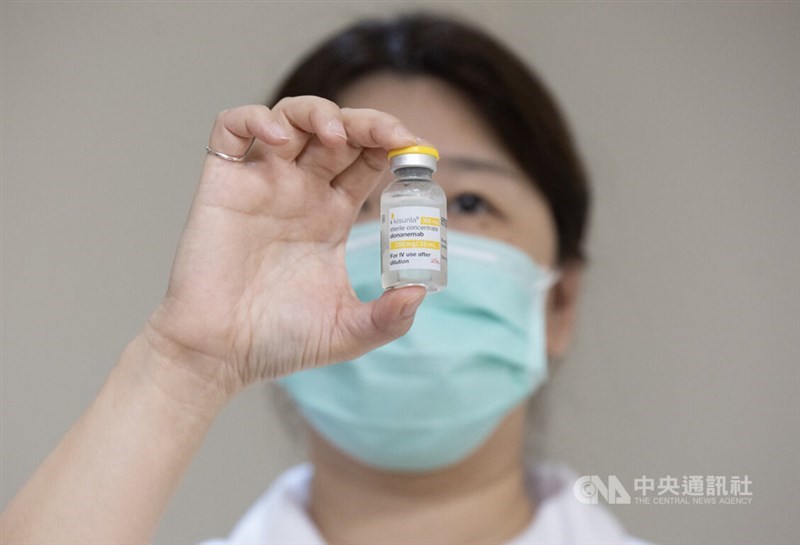
Taipei, June 24 (CNA) Two newly approved Alzheimer's drugs have been administered for the first time in Taiwan, offering new hope to patients in the early stages of the disease, although neither is currently covered by the National Health Insurance (NHI) system.
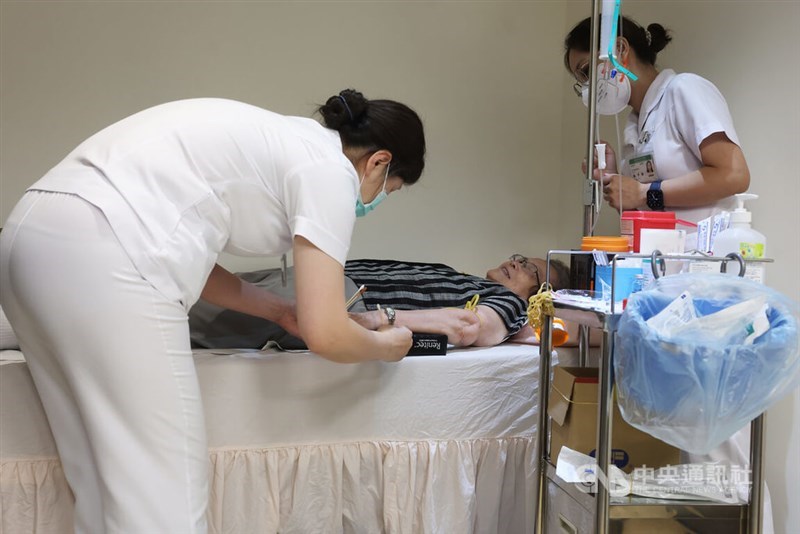
A 76-year-old woman diagnosed with very mild Alzheimer's disease received a dose of Leqembi on Tuesday afternoon, New Taipei Municipal Tucheng Hospital said in a news statement issued the same day.
The administration made the hospital one of the first in Taiwan to offer the newly approved drug, "symbolizing the beginning of a new era in the country's dementia care," the hospital said.
The jab at Tucheng Hospital followed Taiwan's first administration of Kisunla -- another newly approved Alzheimer's drug -- to an 83-year-old female patient at Far Eastern Memorial Hospital (FEMH) in New Taipei on Monday.
Both drugs were approved by the Taiwan Food and Drug Administration (TFDA) earlier in 2025. Leqmebi was approved by the United States Food and Drug Administration in July 2023, while Kisunla got the green light in July 2024.
Kisunla, developed by U.S. pharmaceutical company Eli Lilly, and Leqembi, jointly developed by Eisai in Japan, Biogen in the U.S. and BioArctic in Sweden, are the first new treatments for Alzheimer's disease that target the underlying cause of the disease, rather than just its symptoms, according to the FEMH.
In terms of expected outcomes, nearly 70 percent of patients completely cleared amyloid plaques -- which many researchers believe to be a key cause of Alzheimer's disease due to their abnormal accumulation in the brain -- within a year of receiving the two drugs, the Tucheng Hospital said.
While Leqembi, which requires one injection every two weeks, has the potential to slow the progression of Alzheimer's disease by 27 percent to 51 percent, patients receiving Kisunla -- administered once a month -- may see a 29 percent to 36 percent reduction in disease progression, the Tucheng Hospital added.
Yan Sui-hing (甄瑞興), director of Far Eastern Memorial Hospital's dementia center, told reporters on Monday that the two drugs targeting patients in the early stages of Alzheimer's disease are administered over 18 months of treatment and cost around NT$1.5 million (US$50,829) per course of treatment.
"The cost is a bit high, but we believe it's well worth it," said Mai Ling-chin (麥令琴), whose mother was the first person in Taiwan to receive Kisunla.

During the Monday press event at Far Eastern Memorial Hospital, Mai told reporters that her family felt the treatment was worthwhile if it could help her mother maintain her cognitive abilities and quality of life for a few more years.
An estimated 350,000 in Taiwan have some form of dementia, 60-70 percent of whom have Alzheimer's disease. About half may qualify for the new treatments, totaling fewer than 100,000 patients, according to Yan.
Asked if the two drugs will be covered by the NHI system in the future, National Health Insurance Administration (NHIA) Director-General Shih Chung-liang (石崇良) told reporters on Sunday that a Health Technology Assessment (HTA) will be conducted before making any coverage decisions.
The HTA will evaluate factors such as real-world effectiveness and the potential financial impact on the NHI system, followed by expert reviews and joint committee meetings, Shih said.
He added that the NHIA has so far received only one application from a pharmaceutical company for one of the two new drugs, and if the review process goes smoothly, discussions on NHI coverage could begin by year's end.
- Business
Taiwan October CPI up 1.48%; pork sees biggest rise in 20 months
11/06/2025 09:51 PM - Society
Man sentenced to 4 years for selling banned Sudan Red dye chili powder
11/06/2025 09:29 PM - Society
Four missing after two fishing ships capsize northwest of Taiwan
11/06/2025 08:59 PM - Society
Bag of cash left in train station bathroom leads police to bust fraud ring
11/06/2025 08:48 PM - Society
Taiwan Railway Union launches 'hunger strike' over leave rights
11/06/2025 08:25 PM